

FIRST-LINE ADVANCED/mNSCLC CLINICAL TRIALS SUMMARY
KEYTRUDA: A SUMMARY OF THE CLINICAL TRIALS IN ADVANCED AND mNSCLC
A summary of the clinical trials evaluating the efficacy and safety of KEYTRUDA in certain patients with advanced and metastatic NSCLC: KEYNOTE-024, KEYNOTE-189, KEYNOTE-407 and KEYNOTE-042.

FIRST-LINE MONOTHERAPY
KEYTRUDA FIRST-LINE MONOTHERAPY FOR NON-SQUAMOUS OR SQUAMOUS ADVANCED/mNSCLC
KEYNOTE-042: A multicentre, randomised, controlled trial in patients with Stage III NSCLC who were not candidates for surgical resection or definitive chemoradiation, or patients with metastatic NSCLC. PD-L1 (TPS ≥1%). No EGFR/ALK genomic aberrations.1

FIRST-LINE COMBINATION THERAPY
KEYTRUDA IN COMBINATION WITH CHEMOTHERAPY FOR METASTATIC NON-SQUAMOUS NSCLC
KEYNOTE-189: A multicentre, randomised, active-controlled, double-blind trial of KEYTRUDA + platinum-pemetrexed vs. placebo + platinum-pemetrexed in patients with metastatic non-squamous NSCLC with no EGFR or ALK genomic tumour aberrations.1

FIRST-LINE COMBINATION THERAPY
KEYTRUDA IN COMBINATION WITH CHEMOTHERAPY FOR METASTATIC SQUAMOUS NSCLC
KEYNOTE-407: A randomised, double-blind, multicentre, placebo-controlled study of KEYTRUDA + carboplatin + paclitaxel/nab-paclitaxel vs. placebo + carboplatin + paclitaxel/nab-paclitaxel in patients with metastatic squamous NSCLC.1

ADJUVANT MONOTHERAPY
KEYTRUDA ADJUVANT MONOTHERAPY FOR STAGE IB [T2A ≥4 CM], II, OR IIIA NSCLC)
KEYNOTE-091: A multicentre, randomised, triple-blind, placebo-controlled trial of KEYTRUDA vs. placebo in patients with completely resected Stage IB (T2a ≥4 cm), II, or IIIA NSCLC^ regardless of PD-L1 expression.1
^per AJCC 7th ed
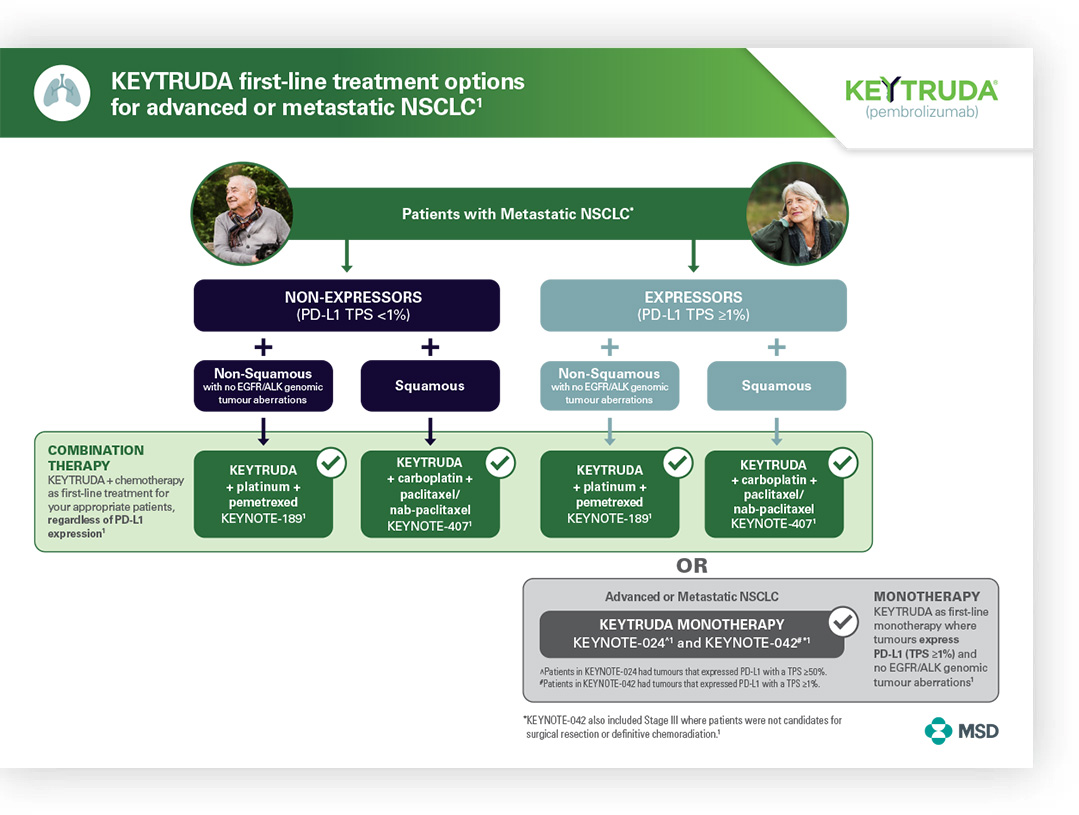
EDUCATIONAL RESOURCE
KEYTRUDA: A FIRST-LINE TREATMENT OPTION IN ADVANCED/mNSCLC
A KEYTRUDA treatment algorithm.
INFORMATION FOR PATIENTS
KEYTRUDA CONSUMER MEDICINE INFORMATION
For more information, refer to the KEYTRUDA Consumer Medicine Information by scanning the QR code or via: www.medsafe.govt.nz/consumers/cmi/k/Keytruda.pdf
INFORMATION FOR HEALTHCARE PROFESSIONALS
KEYTRUDA DATA SHEET
For more information, refer to the KEYTRUDA Data Sheet by scanning the QR code or via: www.medsafe.govt.nz/Profs/DataSheet/k/Keytruda.pdf
KEYTRUDA® (pembrolizumab) is a Prescription Medicine and is available as a 100 mg/4 mL concentrate for solution for infusion.
Please review the KEYTRUDA Data Sheet before prescribing. The Data Sheet is available at www.medsafe.govt.nz.
KEYTRUDA is funded for the first-line treatment of patients with advanced or metastatic NSCLC. Further restrictions apply, see pharmac.govt.nz.2 KEYTRUDA is unfunded for the other NSCLC indications – a charge will apply.
AJCC: American Joint Committee on Cancer; ALK: anaplastic lymphoma kinase; EGFR: epidermal growth factor receptor; mNSCLC: metastatic non-small cell lung cancer; NSCLC: non-small cell lung cancer; PD-L1: programmed death-ligand 1; TPS: tumour proportion score.
References: 1. KEYTRUDA Data Sheet. 2. PHARMAC. Pharmaceutical Schedule. Available at: https://schedule.pharmac.govt.nz/ScheduleOnline.php?edition=&osq=Pembrolizumab Accessed 27 May 2025.
NZ-LAM-00093v5.0. TAPS DA 2422PC. Last updated June 2025.
KEYTRUDA® (pembrolizumab) is a Prescription Medicine and is available as a 100 mg/4 mL concentrate for solution for infusion.
SELECTED SAFETY INFORMATION1
SPECIAL WARNINGS AND PRECAUTIONS FOR USE
Immune-mediated Adverse Reactions
Immune-mediated adverse reactions including severe and fatal cases, have occurred in patients receiving KEYTRUDA. In clinical trials, most immune-mediated adverse reactions occurred during treatment, were reversible and managed with interruptions of KEYTRUDA, administration of corticosteroids and/or supportive care. Immune-related adverse reactions have also occurred after the last dose of KEYTRUDA. Immune-mediated adverse reactions affecting more than one body system can occur simultaneously.
For suspected immune-mediated adverse reactions, ensure adequate evaluation to confirm aetiology or exclude other causes. Based on the severity of the adverse reaction, withhold KEYTRUDA and consider administration of corticosteroids. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Based on limited data from clinical studies in patients whose immune-related adverse reactions could not be controlled with corticosteroid use, administration of other systemic immunosuppressants can be considered. Restart KEYTRUDA if the adverse reaction remains at Grade 1 or less following corticosteroid taper. If another episode of a severe adverse reaction occurs, permanently discontinue KEYTRUDA.
Withhold or discontinue KEYTRUDA to manage adverse reactions as described in the Data Sheet. No dose reductions of KEYTRUDA are recommended.
Immune-mediated pneumonitis
Pneumonitis (including fatal cases) has been reported in patients receiving KEYTRUDA. Monitor patients for signs and symptoms of pneumonitis. If pneumonitis is suspected, evaluate with radiographic imaging and exclude other causes.
Immune-mediated colitis
Colitis has been reported in patients receiving KEYTRUDA. Monitor patients for signs and symptoms of colitis and exclude other causes.
Immune-mediated hepatitis
Hepatitis has been reported in patients receiving KEYTRUDA. Monitor patients for changes in liver function (at the start of treatment, periodically during treatment and as indicated based on clinical evaluation) and symptoms of hepatitis and exclude other causes.
Immune-mediated nephritis
Nephritis has been reported in patients receiving KEYTRUDA. Monitor patients for changes in renal function and exclude other causes.
Immune-mediated endocrinopathies
Adrenal insufficiency (primary and secondary) and hypophysitis has been reported in patients receiving KEYTRUDA. Monitor patients for signs and symptoms of adrenal insufficiency and hypophysitis (including hypopituitarism) and exclude other causes.
Type 1 diabetes mellitus, including diabetic ketoacidosis, has been reported in patients receiving KEYTRUDA. Monitor patients for hyperglycaemia or other signs and symptoms of diabetes.
Thyroid disorders, including hyperthyroidism, hypothyroidism and thyroiditis, have been reported in patients receiving KEYTRUDA and can occur at any time during treatment, therefore monitor patients for changes in thyroid function and clinical signs and symptoms of thyroid disorders.
Severe skin reactions
Immune-mediated severe skin reactions have been reported in patients treated with KEYTRUDA. Monitor patients for suspected severe skin reactions and exclude other causes. Cases of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and bullous pemphigoid have been reported in patients treated with KEYTRUDA. Some cases of SJS and TEN have had a fatal outcome.
Other immune-mediated adverse reactions
The following additional clinically significant, immune-mediated adverse reactions were reported in less than 1% of patients treated with KEYTRUDA in KEYNOTE-001, KEYNOTE-002, KEYNOTE-006, and KEYNOTE-010: uveitis, myositis, Guillain-Barre syndrome, pancreatitis, encephalitis, sarcoidosis, myasthenic syndrome/myasthenia gravis (including exacerbation), myelitis, vasculitis, hypoparathyroidism, gastritis, haemolytic anaemia and pericarditis. The following was reported in other clinical studies with KEYTRUDA or in post-marketing use: myocarditis, sclerosing cholangitis and exocrine pancreatic insufficiency.
Transplant-related adverse reactions
Solid organ transplant rejection has been reported in the post-marketing setting in patients treated with KEYTRUDA. Treatment with KEYTRUDA may increase the risk of rejection in solid organ transplant recipients. Consider the benefit of treatment with KEYTRUDA versus the risk of possible organ rejection in these patients.
Acute graft-versus-host-disease (GVHD), including fatal GVHD, after treatment with KEYTRUDA has been reported in patients with a history of allogeneic hematopoietic stem cell transplant (HSCT). Patients who experienced GVHD after their transplant procedure may be at increased risk for GVHD after treatment with KEYTRUDA. Consider the benefit of treatment with KEYTRUDA versus the risk of possible GVHD in patients with a history of allogeneic HSCT.
Elevated liver enzymes when KEYTRUDA is given in combination with axitinib for RCC
When KEYTRUDA is given with axitinib, higher than expected frequencies of Grades 3 and 4 ALT and AST elevations have been reported in patients with advanced RCC. Monitor liver enzymes before initiation of and periodically throughout treatment. Consider more frequent monitoring of liver enzymes as compared to when the drugs are used in monotherapy. Follow medical management guidelines for both drugs. Refer to the prescribing information for axitinib.
Increased mortality in patients with multiple myeloma when KEYTRUDA is added to a thalidomide analogue and dexamethasone
In two randomised clinical trials in patients with multiple myeloma, the addition of KEYTRUDA to a thalidomide analogue plus dexamethasone, a use for which no PD-1 or PD-L1 blocking antibody is indicated, resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
Infusion-related reactions
Severe infusion reactions, including hypersensitivity and anaphylaxis, have been reported in 6 (0.2%) of 2799 patients receiving KEYTRUDA in KEYNOTE-001, KEYNOTE-002, KEYNOTE-006 and KEYNOTE-010. Patients with mild or moderate infusion reaction may continue to receive KEYTRUDA with close monitoring; premedication with antipyretic and antihistamine may be considered.
Patients excluded from clinical trials
Patients with HIV, HBV, HCV, other active infections requiring therapy; and patients with a history of severe immune-mediated adverse reactions with ipilimumab, defined as any Grade 4 toxicity requiring treatment with corticosteroids or Grade 3 toxicity requiring corticosteroid treatment (greater than 10 mg/day prednisone or equivalent dose) for greater than 12 weeks were excluded from the clinical trial. No clinical data is available. Caution should be used in these patient populations.
Patients who experienced less severe adverse reactions (including immune-mediated) on ipilimumab that resolved or improved to Grade 0-1 and ≤10 mg/day prednisone (or equivalent dose) for immune-mediated adverse events for at least two weeks prior to first dose of KEYTRUDA were included in the clinical trial. Caution should be used in this patient population.
Effect on Laboratory Tests
Thyroid and liver (hepatic transaminase and bilirubin levels) function tests should be performed at the start of treatment, periodically during treatment and as indicated based on clinical evaluation.
Effects on Fertility
Fertility studies have not been conducted with KEYTRUDA.
Use in Pregnancy (Category D) and Use in Lactation
There are no data on the use of KEYTRUDA in pregnant women. KEYTRUDA is not recommended during pregnancy unless the clinical benefit outweighs the potential risk to the foetus. Women of childbearing potential should use effective contraception during treatment with KEYTRUDA and for at least 4 months following the last dose of KEYTRUDA.
It is unknown whether KEYTRUDA is secreted in human milk. A decision should be made whether to discontinue breast-feeding or to discontinue KEYTRUDA.
Effects on ability to drive and use machines
Because of potential adverse reactions such as fatigue, patients should be advised to use caution when driving or operating machinery until they are certain that KEYTRUDA does not adversely affect them.
CONTRAINDICATIONS
Hypersensitivity to the active substance or to any of the excipients; Histidine, Histidine monohydrochloride monohydrate, Sucrose, Polysorbate 80 and water for injection.
INTERACTIONS WITH OTHER MEDICINES
No formal pharmacokinetic drug interaction studies have been conducted with KEYTRUDA. The use of systemic corticosteroids or immunosuppressants before starting KEYTRUDA should be avoided, however, systemic corticosteroids or other immunosuppressants can be used after starting KEYTRUDA to treat immune-mediated adverse reactions. Corticosteroids can also be used as premedication, when Keytruda is used in combination with chemotherapy, as antiemetic prophylaxis and/or to alleviate chemotherapy-related adverse reactions.
ADVERSE EFFECTS
Clinical trials experience
The safety of KEYTRUDA was evaluated in 2799 patients with melanoma and NSCLC in controlled and uncontrolled studies. The median treatment duration was 4.2 months (range 1 day to 30.4 months) including 1153 patients treated for greater than or equal to six months and 600 patients treated for greater than or equal to one year.
KEYTRUDA was discontinued for treatment-related adverse reactions in 5% of patients. Treatment-related serious adverse events (SAEs) reported up to 90 days after the last dose occurred in 10% of patients receiving KEYTRUDA. Of these treatment-related SAEs, the most common were: pneumonitis, colitis, diarrhoea, and pyrexia. The most common treatment-related adverse reactions (reported in >10% of patients) were: fatigue, pruritus, rash, diarrhoea, and nausea.
Immune-mediated adverse reactions
Immune-mediated adverse reactions are presented based on 2799 patients with melanoma and NSCLC. Table 1 presents the incidence of immune-mediated adverse reactions by Grade that occurred in patients receiving KEYTRUDA.
Table 1. Immune-Mediated Adverse Reactions
KEYTRUDA
2 mg/kg every 3 weeks or 10 mg/kg every 2 or 3 weeks, n=2799
| ADVERSE REACTION | All Grades (%) | Grades 2 (%) | Grades 3 (%) | Grades 4 (%) | Grades 5 (%) |
|---|---|---|---|---|---|
| Hypothyroidism* | 8.5 | 6.2 | 0.1 | 0 | 0 |
| Hyperthyroidism † | 3.4 | 0.8 | 0.1 | 0 | 0 |
| Pneumonitis ‡ | 3.4 | 1.3 | 0.9 | 0.3 | 0.1 |
| Colitis | 1.7 | 0.4 | 1.1 | <0.1 | 0 |
| Adrenal Insufficiency | 0.8 | 0.3 | 0.3 | <0.1 | 0 |
| Hepatitis | 0.7 | 0.1 | 0.4 | <0.1 | 0 |
| Hypophysitis | 0.6 | 0.2 | 0.3 | <0.1 | 0 |
| Nephritis § | 0.3 | 0.1 | 0.1 | <0.1 | 0 |
| Type 1 Diabetes Mellitus | 0.2 | <0.1 | 0.1 | 0.1 | 0 |
*In patients with cHL (n=389) the incidence of hypothyroidism was 17%, all of which were Grade 1 or 2. In individual studies of patients with HNSCC treated with KEYTRUDA as monotherapy (n=909) the incidence of hypothyroidism was 16.1% (all Grades) with 0.3% Grade 3. In patients with HNSCC treated with KEYTRUDA in combination with platinum and 5-FU chemotherapy (n=276) the incidence of hypothyroidism was 15.2%, all of which were Grade 1 or 2. In the adjuvant study of patients with resected RCC treated with KEYTRUDA as monotherapy (n=488) the incidence of hypothyroidism was 21% (all Grades) with 0.2% Grade 3.
† In the adjuvant study of patients with resected RCC treated with KEYTRUDA as monotherapy (n=488) the incidence of hyperthyroidism was 12% (all Grades) with 0.2% Grade 3.
‡In individual studies of patients with NSCLC treated with KEYTRUDA as monotherapy (total n=2602), the incidence of pneumonitis (all Grades) ranged from 3.8% to 8.3%. In cHL patients treated with KEYTRUDA as monotherapy, the incidence of pneumonitis (all Grades) ranged from 5.2% to 10.8% for cHL patients in KEYNOTE-087 (n=210) and KEYNOTE-204 (n=148), respectively.
§In patients with non-squamous NSCLC treated with KEYTRUDA 200 mg in combination with pemetrexed and platinum chemotherapy (n=405) the incidence of nephritis was 1.7% (all Grades) with 1.0% Grade 3 and 0.5% Grade 4.
Other Adverse Events
Melanoma
In KEYNOTE-006, adverse events with KEYTRUDA in treatment-naïve patients with advanced melanoma occurring in ≥10% and at a higher incidence than with ipilimumab (between arm difference of ≥5% [All Grades] or ≥2% [Grade 3]) were arthralgia, cough, back pain, and vitiligo.
In KEYNOTE-006, worsening laboratory abnormalities with KEYTRUDA occurring in ≥20% of patients and at a higher incidence than with ipilimumab (between arm difference of ≥5% [All Grades] or ≥2% [Grade 3-4]) were lymphopaenia and hypertriglyceridaemia.
In KEYNOTE-002, adverse events with KEYTRUDA in patients previously treated with ipilimumab for advanced melanoma occurring in ≥10% and at a higher incidence than with chemotherapy (between arm difference of ≥5% [All Grades] or ≥2% [Grade 3]) were pruritis, arthralgia, abdominal pain, rash and hyponatraemia.
In KEYNOTE-002, worsening laboratory abnormalities with KEYTRUDA occurring in ≥20% of patients and at a higher incidence than with chemotherapy (between arm difference of ≥5% [All Grades] or ≥2% [Grade 3-4]) were anaemia, hyperglycaemia, hyponatraemia, hypoalbuminaemia, increased aspartate aminotransferase and increased alkaline phosphatase.
Among the 969 patients with resected melanoma enrolled in KEYNOTE-716 and 1019 patients with resected melanoma enrolled in KEYNOTE-054, the adverse reactions were generally similar to those occurring in patients with advanced melanoma or NSCLC.
Non-Small Cell Lung Carcinoma
In KEYNOTE-189, adverse events with KEYTRUDA in combination with pemetrexed and platinum chemotherapy in previously untreated patients with metastatic non-squamous NSCLC occurring in ≥20% and at a higher incidence than with placebo with pemetrexed and platinum chemotherapy (between arm difference of ≥5% [All Grades] or ≥2% [Grade 3-4]) were fatigue, diarrhoea, neutropenia, rash and asthenia.
Adverse events occurring in previously untreated patients with metastatic squamous NSCLC receiving KEYTRUDA in combination with carboplatin and either paclitaxel or nab-paclitaxel in KEYNOTE-407 were generally similar to those occurring in patients in KEYNOTE-189 with the exception of alopecia (46%) and arthralgia (21%).
In KEYNOTE-042, adverse events with KEYTRUDA in previously untreated patients with NSCLC occurring in ≥10% of patients and at a higher incidence than with chemotherapy (between arm difference of ≥5% [All Grades] or ≥2% [Grade 3-5]) were dyspnoea, cough and hypothyroidism.
Adverse events occurring in previously untreated patients with NSCLC receiving KEYTRUDA in KEYNOTE- 024 and previously treated patients in KEYNOTE-010 were generally similar to those occurring in patients in KEYNOTE-042.
Among the 580 patients with resected NSCLC treated with KEYTRUDA in KEYNOTE-091, the adverse reactions were generally similar to those occurring in other patients with NSCLC receiving KEYTRUDA as monotherapy with the exception of hypothyroidism (21%) and hyperthyroidism (11%).
Adverse events occurring in patients with resectable NSCLC receiving KEYTRUDA in combination with platinum-containing chemotherapy, given as neoadjuvant treatment and continued as monotherapy adjuvant treatment in KEYNOTE-671, were generally similar to those occurring in patients in other clinical trials across tumor types receiving KEYTRUDA in combination with chemotherapy.
Head and Neck Squamous Cell Cancer
In patients with HNSCC receiving KEYTRUDA plus chemotherapy (platinum and 5-FU), adverse reactions occurring at a greater severity (Grade 3-4) and at a higher incidence (≥2% difference) compared to cetuximab plus chemotherapy (platinum and 5-FU) were: fatigue (7%), mucosal inflammation (10%), and stomatitis (8%).
Adverse events occurring with KEYTRUDA monotherapy in patients with HNSCC were generally similar to those occurring in patients with melanoma or NSCLC.
Gastric Cancer
In patients with gastric cancer receiving KEYTRUDA plus trastuzumab and chemotherapy (fluoropyrimidine and platinum), adverse events occurring in at least 20% of patients and at a higher incidence (≥2% difference) of Grades 3-4 severity compared to placebo plus trastuzumab and chemotherapy (fluoropyrimidine and platinum) were: vomiting (4.6% vs. 1.9%), anaemia (14% vs.12%), decreased platelet count (14% vs.10%), and lymphopenia (13% vs.9%).
In patients with gastric cancer receiving KEYTRUDA plus chemotherapy (fluoropyrimidine and platinum), adverse events occurring in at least 20% of patients and at a higher incidence (≥2% difference) of Grades 3-4 severity compared to placebo plus chemotherapy (fluoropyrimidine and platinum) were: anaemia (12% vs. 10%), platelet count decreased (7% vs. 5%).
Cervical Cancer
In patients with cervical cancer receiving KEYTRUDA plus chemotherapy (paclitaxel and cisplatin or paclitaxel and carboplatin) with or without bevacizumab, adverse reactions occurring at a higher incidence (≥2%) of Grades 3-5 severity for KEYTRUDA plus chemotherapy with or without bevacizumab compared to placebo plus chemotherapy with or without bevacizumab were: anaemia (30% vs. 27%), neutropenia (12% vs. 10%), thrombocytopenia (8% vs. 5%), asthenia (3.6% vs. 1.6%).
Renal Cell Carcinoma
In KEYNOTE-426, the most common adverse reactions that occurred in ≥20% of previously untreated patients with RCC receiving KEYTRUDA and axitinib were diarrhoea, hypertension, fatigue, hypothyroidism, decreased appetite, palmar-plantar erythrodysaesthesia syndrome, nausea, ALT increased, AST increased, dysphonia, cough and constipation. In KEYNOTE-426, a higher than expected incidence of Grades 3 and 4 ALT increased (20%) and AST increased (13%) were observed in previously untreated patients with RCC receiving KEYTRUDA in combination with axitinib.
In KEYNOTE-581, the most common adverse events occurring in ≥20% of patients with receiving KEYTRUDA with lenvatinib and at a higher incidence than with sunitinib (between arm difference of ≥5% [All Grades] or ≥2% [Grades 3-4]) were diarrhoea, nausea, vomiting, constipation, abdominal pain, hypertension, hypothyroidism, decreased appetite, dysphonia, decreased weight, proteinuria, rash, arthralgia and headache.
Adverse events occurring in patients receiving KEYTRUDA as adjuvant treatment in RCC were generally similar to those occurring in patients with melanoma or NSCLC.
Endometrial Cancer
In KEYNOTE-868, patients with endometrial carcinoma receiving KEYTRUDA plus chemotherapy (paclitaxel and carboplatin) experienced adverse events that generally similar to those observed with KEYTRUDA alone or chemotherapy alone with the exception of rash maculo-papular (13% all Grades; 2% Grades 3-4).
In KEYNOTE-775, adverse events occurring in ≥20% of patients receiving KEYTRUDA with lenvatinib and at a higher incidence than with chemotherapy (between arm difference of ≥5% [All Grades] or ≥2% [Grades 3-4]) were hypertension, hypothyroidism, diarrhoea, nausea, vomiting, abdominal pain, decreased appetite, decreased weight, increased ALT, fatigue, asthenia, arthralgia, proteinuria, urinary tract infection, headache, dysphonia, and palmar-plantar erythrodysaesthesia syndrome. There was one Grade 5 adverse event (0.2%) reported. Refer to the Lenvatinib Data Sheet for Lenvatinib discontinuation and interruption information.
Oesophageal Cancer
In patients with oesophageal cancer, adverse reactions occurring in ≥20% of patients and at a higher incidence (≥2%) of Grades 3-5 severity for KEYTRUDA in combination with chemotherapy (cisplatin and 5-FU) compared to placebo and chemotherapy (cisplatin and 5-FU) were: vomiting (7%), stomatitis (6%), neutrophil count decreased (24.1%), and white blood cell count decreased (9.2%).
Triple-Negative Breast Cancer
In KEYNOTE-522, adverse reactions occurring in at least 20% of patients with high-risk early-stage TNBC receiving KEYTRUDA in combination with chemotherapy (carboplatin and paclitaxel followed by doxorubicin or epirubicin and cyclophosphamide), and at a higher incidence (≥5% difference) compared to patients receiving placebo in combination with chemotherapy (carboplatin and paclitaxel followed by doxorubicin or epirubicin and cyclophosphamide), were diarrhoea, rash, pyrexia, and decreased appetite.
In KEYNOTE-355 adverse reactions occurring in at least 20% of the patients with locally recurrent unresectable or metastatic TNBC receiving KEYTRUDA in combination with chemotherapy (paclitaxel, nab-paclitaxel, or gemcitabine and carboplatin), and at a higher incidence (≥5% difference) compared to patients receiving placebo in combination with chemotherapy (paclitaxel, nab-paclitaxel, or gemcitabine and carboplatin) were diarrhoea, decreased appetite, and rash.
Biliary Tract Carcinoma
In patients with BTC receiving KEYTRUDA plus chemotherapy (gemcitabine and cisplatin), adverse events occurring at a higher incidence (≥5%) compared to placebo plus chemotherapy were pyrexia (26% vs. 20%), rash (17% vs. 9%), pruritus (15% vs. 10%) and hypothyroidism (9% vs. 2.6%). Of these adverse events, Grades 3-4 events were pyrexia (2.3% vs. 0.9%), rash (0.6% vs. 0.4%), pruritus (0.0% vs. 0.0%) and hypothyroidism (0.2% vs 0.0%).
Merkel Cell Carcinoma
Among the 105 patients with MCC enrolled in KEYNOTE-017 and KEYNOTE-913, the adverse reactions were similar to those occurring in 2799 patients with melanoma or NSCLC treated with KEYTRUDA as a single agent. Laboratory abnormalities (Grades 3-4) that occurred at a higher incidence included increased lipase (17%).
Malignant Pleural Mesothelioma
Among the 241 patients with MPM treated with KEYTRUDA in combination with pemetrexed and platinum chemotherapy in KEYNOTE-483, the adverse events were generally similar to those occurring in other patients receiving KEYTRUDA in combination with pemetrexed and platinum chemotherapy.
Other Cancers - Monotherapy
Adverse events occurring in patients with urothelial carcinoma, cHL, MSI-H/dMMR cancer, CRC, or recurrent or metastatic cSCC, as well as locally advanced cSCC, were generally similar to those occurring in patients with melanoma or NSCLC.
Paediatric Patients
In KEYNOTE-051, 161 paediatric patients (62 children ages 6 months to less than 12 years and 99 adolescents ages 12 years to 17 years) with advanced melanoma, lymphoma, or PD-L1 positive or MSI-H advanced, relapsed, or refractory solid tumours were administered KEYTRUDA 2 mg/kg every 3 weeks. Patients received KEYTRUDA for a median of 4 doses (range 1-35 doses), with 138 patients (86%) receiving KEYTRUDA for 2 doses or more. The concentrations of KEYTRUDA in paediatric patients were comparable to those observed in adult patients at the same dose regimen of 2 mg/kg every 3 weeks.
The safety profile in those paediatric patients was similar to that seen in adults treated with pembrolizumab. The most common adverse reactions (reported in ≥20% of paediatric patients) were pyrexia, vomiting, headache, abdominal pain, anaemia, cough, and constipation.
INDICATIONS1
Melanoma
KEYTRUDA is indicated as monotherapy for the treatment of unresectable or metastatic melanoma in adults.
KEYTRUDA is indicated for the adjuvant treatment of adult and paediatric (12 years and older) patients with Stage IIB, IIC, or III melanoma who have undergone complete resection.
Non-small cell lung cancer
KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of patients with metastatic non-squamous non-small cell lung carcinoma (NSCLC), with no EGFR or ALK genomic tumour aberrations.
KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of patients with metastatic squamous NSCLC.
KEYTRUDA as monotherapy is indicated for the first-line treatment of patients with NSCLC expressing PD-L1 [tumour proportion score (TPS) ≥1%] as determined by a validated test, with no EGFR or ALK genomic tumour aberrations, and is
- stage III where patients are not candidates for surgical resection or definitive chemoradiation, or
- metastatic.
KEYTRUDA as monotherapy is indicated for the treatment of patients with advanced NSCLC whose tumours express PD-L1 with a ≥1% TPS as determined by a validated test and who have received platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumour aberrations should have received prior therapy for these aberrations prior to receiving KEYTRUDA.
KEYTRUDA is indicated for the treatment of patients with resectable Stage II, IIIA, or IIIB (T3-4N2) NSCLC in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment.
KEYTRUDA as monotherapy is indicated for the adjuvant treatment of patients with Stage IB (T2a ≥4 cm), II, or IIIA NSCLC who have undergone complete resection.
Classical Hodgkin Lymphoma
KEYTRUDA is indicated for the treatment of adult and paediatric patients with relapsed or refractory classical Hodgkin Lymphoma (cHL).
Urothelial Carcinoma
KEYTRUDA is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumours express PD-L1 [Combined Positive Score (CPS) ≥10] as determined by a validated test, or in patients who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status.
KEYTRUDA is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have received platinum-containing chemotherapy.
KEYTRUDA is indicated for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in-situ with or without papillary tumours who are ineligible for or have elected not to undergo cystectomy.
Head & Neck Squamous Cell Cancer
KEYTRUDA, in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the first-line treatment of patients with metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC).
KEYTRUDA, as monotherapy, is indicated for the first-line treatment of patients with metastatic or unresectable recurrent HNSCC, whose tumours express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by a validated test.
KEYTRUDA, as monotherapy, is indicated for the treatment of patients with metastatic or unresectable recurrent HNSCC with disease progression on or after platinum-containing chemotherapy.
Microsatellite instability-high cancer
Colorectal
KEYTRUDA is indicated in adult and paediatric patients for the treatment of unresectable or metastatic, microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. This indication was approved based on objective response rate and response duration in a single-arm trials.
Non-colorectal
KEYTRUDA is indicated in adult and paediatric patients for the treatment of unresectable or metastatic, MSI-H or dMMR tumours that have progressed following prior treatment and when there are no satisfactory alternative treatment options. Sample sizes for individual tissue types were too small to provide data on clinical utility of the MSI-H/dMMR tests for each of the tissue types, individually. The assumption that MSI-H/dMMR-status is predictive of the treatment effect of KEYTRUDA for every tissue type has not been verified.
The safety and effectiveness of KEYTRUDA in paediatric patients with MSI-H central nervous system cancers have not been established.
Colorectal Cancer
KEYTRUDA is indicated for the first-line treatment of patients with unresectable or metastatic MSI-H or dMMR colorectal cancer (CRC).
Endometrial Carcinoma
KEYTRUDA, in combination with carboplatin and paclitaxel, followed by KEYTRUDA as a single agent, is indicated for the treatment of adult patients with primary advanced or recurrent endometrial carcinoma.
KEYTRUDA, in combination with lenvatinib, is indicated for the treatment of patients with advanced endometrial carcinoma, who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation.
Cervical Cancer
KEYTRUDA, in combination with platinum chemotherapy and paclitaxel, with or without bevacizumab, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer whose tumours express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by a validated test.
Cutaneous Squamous Cell Carcinoma
KEYTRUDA is indicated for the treatment of patients with recurrent or metastatic cutaneous squamous cell carcinoma (cSCC) or locally advanced cSCC that is not curable by surgery or radiation.
Renal Cell Carcinoma
KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of patients with advanced renal cell carcinoma (RCC).
KEYTRUDA, in combination with lenvatinib, is indicated for the first-line treatment of patients with advanced RCC.
KEYTRUDA, as monotherapy, is indicated for the adjuvant treatment of patients with RCC at intermediate-high or high risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.
Oesophageal Cancer
KEYTRUDA, in combination with platinum and fluoropyrimidine based chemotherapy, is indicated for the first-line treatment of patients with locally advanced unresectable or metastatic carcinoma of the oesophagus or gastroesophageal junction.
Triple-Negative Breast Cancer
KEYTRUDA is indicated for the treatment of patients with high-risk early-stage triple-negative breast cancer (TNBC) in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery.
KEYTRUDA in combination with chemotherapy, is indicated for the treatment of patients with locally recurrent unresectable or metastatic TNBC whose tumours express PD-L1 (CPS ≥10) as determined by a validated test.
Gastric Cancer
KEYTRUDA, in combination with trastuzumab, fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the first-line treatment of patients with locally advanced unresectable or metastatic HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma, whose tumours express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by a validated test.
KEYTRUDA, in combination with fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the first-line treatment of patients with locally advanced unresectable or metastatic HER2-negative gastric or GEJ adenocarcinoma.
Merkel Cell Carcinoma
KEYTRUDA, as monotherapy, is indicated for the treatment of adult and paediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma (MCC).
Biliary Tract Carcinoma
KEYTRUDA, in combination with gemcitabine and cisplatin, is indicated for the treatment of patients with locally advanced unresectable or metastatic biliary tract carcinoma (BTC).
Malignant Pleural Mesothelioma
KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of adult and paediatric (12 years and older) patients with unresectable advanced or metastatic malignant pleural mesothelioma (MPM).
DOSAGE AND ADMINISTRATION1
Recommended Dosing
KEYTRUDA is administered as an intravenous infusion over 30 minutes. The recommended dose of KEYTRUDA in adults is:
- 200 mg every 3 weeks or
- 400 mg every 6 weeks
The recommended dose of KEYTRUDA in paediatric patients is 2 mg/kg (up to a maximum of 200 mg) every 3 weeks.
For use in combination, please review the Data Sheets for KEYTRUDA and the relevant concomitant therapies. When administering KEYTRUDA as part of a combination with intravenous chemotherapy, KEYTRUDA should be administered first.
Patients should be treated with KEYTRUDA until disease progression or unacceptable toxicity. Atypical responses (i.e., an initial transient increase in tumour size or small new lesions within the first few months followed by tumour shrinkage) have been observed. Clinically stable patients with initial evidence of disease progression can under some circumstances remain on treatment until disease progression is confirmed.
For the adjuvant treatment of melanoma, RCC or NSCLC, KEYTRUDA should be administered for up to one year or until disease recurrence or unacceptable toxicity.
For the neoadjuvant and adjuvant treatment of resectable NSCLC, patients should be treated with neoadjuvant KEYTRUDA in combination with chemotherapy for 12 weeks or until disease progression that precludes definitive surgery or unacceptable toxicity, followed by adjuvant treatment with KEYTRUDA as monotherapy for 39 weeks or until disease recurrence or unacceptable toxicity.
For the neoadjuvant and adjuvant treatment of high-risk early-stage TNBC, patients should be treated with neoadjuvant KEYTRUDA in combination with chemotherapy for 8 doses of 200 mg every 3 weeks or 4 doses of 400 mg every 6 weeks or until disease progression that precludes definitive surgery or unacceptable toxicity, followed by adjuvant treatment with KEYTRUDA as monotherapy for 9 doses of 200 mg every 3 weeks or 5 doses of 400 mg every 6 weeks or until disease recurrence or unacceptable toxicity. Patients who experience disease progression that precludes definitive surgery or unacceptable toxicity related to KEYTRUDA as neoadjuvant treatment in combination with chemotherapy should not receive KEYTRUDA monotherapy as adjuvant treatment.
No dose reductions of KEYTRUDA are recommended.
Use in Geriatric Patients
No overall differences in safety or efficacy were reported between elderly patients (65 years and over) and younger patients (less than 65 years). No dose adjustment is necessary in this population.
Use in patients with Renal/Hepatic Insufficiency
No dose adjustment is needed for patients with mild or moderate renal impairment, or mild hepatic impairment. KEYTRUDA has not been studied in patients with severe renal impairment, or moderate or severe hepatic impairment.
Please review the KEYTRUDA Data Sheet before prescribing. The Data Sheet is available at www.medsafe.govt.nz or by clicking on ‘Data Sheet’ on the menu bar of this website.
KEYTRUDA is funded for the treatment of eligible patients with: Stage IIIB-D melanoma, advanced melanoma, advanced MSI-H or dMMR colorectal cancer, relapsed or refractory cHL, previously treated advanced urothelial carcinoma. KEYTRUDA is funded for the first-line treatment of eligible patients with the following advanced cancers: NSCLC, HNSCC, and TNBC. Further restrictions apply, see www.pharmac.govt.nz.2 KEYTRUDA is unfunded for all other indications - a charge will apply.
References:
- KEYTRUDA Data Sheet.
- PHARMAC. Pharmaceutical Schedule. Available at: https://schedule.pharmac.govt.nz/ScheduleOnline.php?edition=&osq=pembrolizumab Accessed 17 July 2025.
NZ-KEY-00834v16.0 TAPS DA 2419KN. Last updated September 2025.


